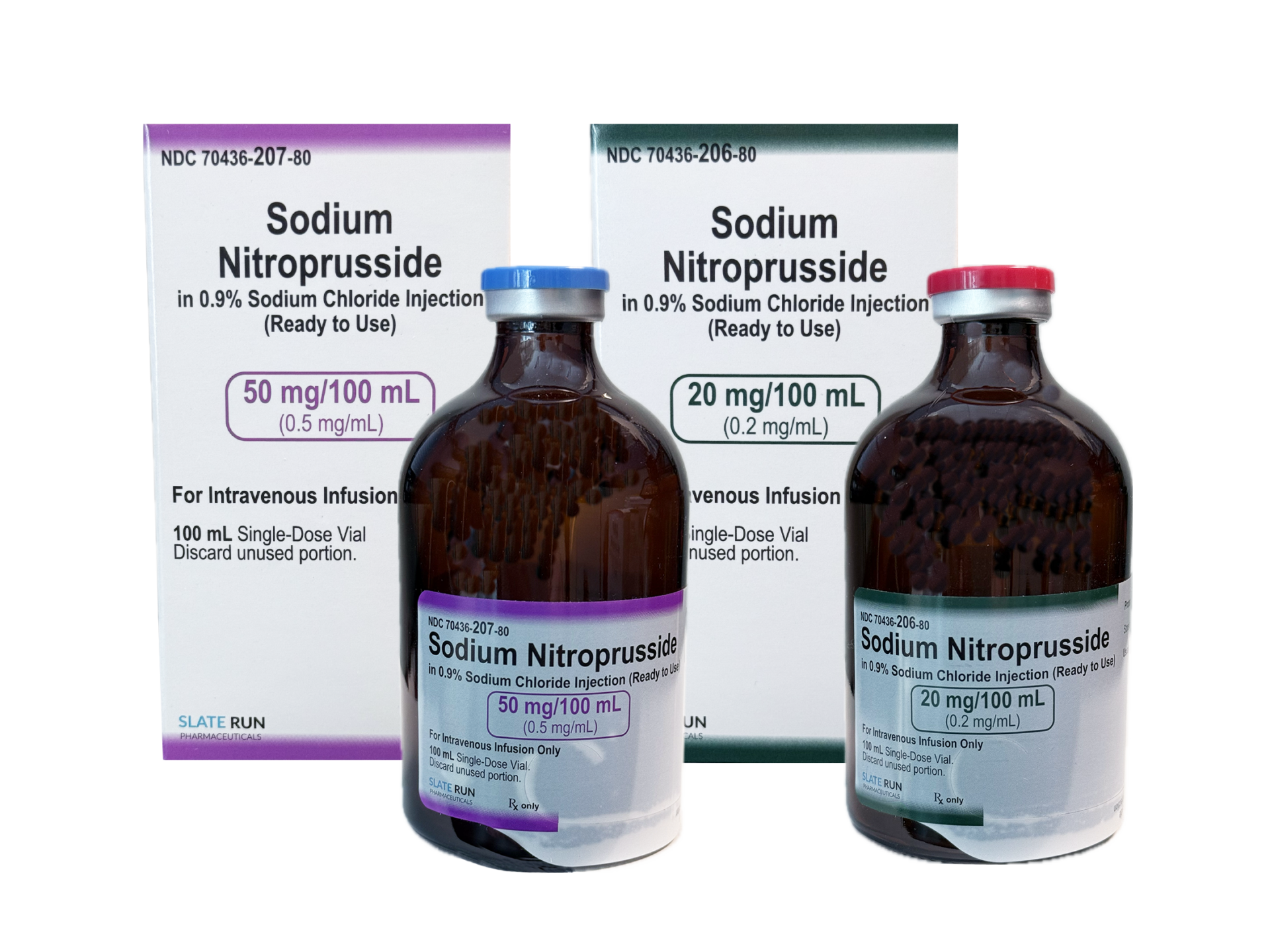
SODIUM NITROPRUSSIDE
in 0.9% Sodium Chloride INJECTION
PRODUCT DETAILS
-
Concentration:
20 mg / 100 mL (0.2 mg / mL)
50 mg / 100 mL (0.5 mg / mL) -
Fill Volume: Sterile Solution in a 100 mL vial
-
Therapeutic Class: Vasodilator
-
Closure does not contain any natural rubber or latex
-
Preservative free
NDC Numbers:
20 mg / 100 mL (0.2 mg / mL) | Box of 1 vial (100 mL): 70436-206-80
50 mg / 100 mL (0.5 mg / mL) | Box of 1 vial (100 mL): 70436-207-80
Available through your wholesaler:
NDC: 70436-206-80
Cardinal Health: 5906334
McKesson: 2909174
Amerisource: 10287055
NDC: 70436-207-80
Cardinal Health: 5906342
McKesson: 2909166
Amerisource: 10287033
| Strength | NDC | Saleable Unit |
|---|---|---|
| 20 mg/100 mL (0.2 mg/mL) | 70436-206-80 | Box of 1 vial Sterile Solution (100 mL) |
| 50 mg/100 mL (0.5 mg/mL) | 70436-207-80 | Box of 1 vial Sterile Solution (100 mL) |
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature].
To protect Sodium Nitroprusside in 0.9% Sodium Chloride Injection, ready to use from light, vial should be stored in its carton until used.
IMPORTANT SAFETY INFORMATION FOR:
SODIUM NITROPRUSSIDE IN 0.9% SODIUM CHLORIDE INJECTION
WARNING: (A) EXCESSIVE HYPOTENSION; (B) CYANIDE TOXICITY
(A) EXCESSIVE HYPOTENSION: Sodium nitroprusside can cause precipitous decreases in blood pressure which can lead to irreversible ischemic injuries or death. Use only with continuous blood pressure monitoring.
(B) CYANIDE TOXICITY: Sodium nitroprusside metabolism produces dose-related cyanide, which can be lethal. A patient’s ability to buffer cyanide will be exceeded in less than one hour at the maximum dose rate (10 mcg/kg/min); limit infusion at the maximum rate to as short a duration as possible.
Sodium Nitroprusside in 0.9% Sodium Chloride Injection is contraindicated in:
- Diseases of compensatory hypertension (e.g., coarctation of the aorta, arteriovenous shunting).
- Inadequate cerebral circulation or in moribund patients (A.S.A. Class 5E) coming to emergency surgery.
- Patients with congenital (Leber’s) optic atrophy or with tobacco amblyopia.
- Acute heart failure associated with reduced peripheral vascular resistance.
- Concomitant use with sildenafil, tadalafil, vardenafil, or riociguat.
Most of the cyanide produced during metabolism of sodium nitroprusside is eliminated in the form of thiocyanate. Thiocyanate is mildly neurotoxic (tinnitus, miosis, hyperreflexia) at serum levels of 1 mmol/L (60 mg/L). Thiocyanate is life-threatening when levels reach ~200 mg/L. Therefore, routine monitoring of plasma thiocyanate levels is recommended in patients with normal renal function when cumulative sodium nitroprusside doses exceed 7 mg/kg/day. In patients with eGFR <30 mL/min/1.73 m 2, limit the mean infusion rate to less than 3 mcg/kg/min. In anuric patients, limit the mean infusion rate to 1 mcg/kg/min.
Sodium nitroprusside infusions cause conversion of hemoglobin to methemoglobin in a dose- dependent manner. Suspect methemoglobinemia in patients who have received >10 mg/kg of sodium nitroprusside and who exhibit signs of impaired oxygen delivery despite adequate cardiac output and adequate arterial pO2. Methemoglobinemic blood is chocolate brown, without the expected color change on exposure to air. Methemoglobin levels >10% are considered clinically significant.
When methemoglobinemia is diagnosed, the treatment of choice is 1-2 mg/kg of methylene blue, administered intravenously over several minutes.
Like other vasodilators, sodium nitroprusside can cause increases in intracranial pressure.
When sodium nitroprusside (or any other vasodilator) is used for controlled hypotension during anesthesia, the patient’s capacity to compensate for anemia and hypovolemia may be diminished. If possible, correct pre-existing anemia and hypovolemia prior to administration.
The most common adverse reactions are hypotension and cyanide toxicity.
Please see the package insert for Sodium Nitroprusside in 0.9% Sodium Chloride Injection for full prescribing information.